Aging and Mitochondria (Premature Aging)
Some Premature Aging Causes Have Been Identified and These Can Be Easily Changed by Anyone Interested in Staying as “Young” and Healthy as Possible
There are many theories of aging. Some involve processes over which we presently have no control and a huge number yet undiscovered. Out of this highly contentious issue arose a theory involving mitochondria in our cells, which shows the greatest promise to modify, reverse, and “cure” degenerative diseases. Mitochondrial Medicine may prove to be the wave of the future for medicine. It is already proving of benefit in returning homeostasis to “disorders” in the body. Mitochondrial impairment has been linked to the degenerative diseases of normal aging and because these same impairments are also being found in younger aged people they are thought to be the cause of “premature aging” and therefore why younger people are developing these diseases of the aged. Identified factors that lead to mitochondrial impairment just happen to be factors that are adaptable to change by ourselves and with the help of sophisticated metabolic testing.
The Degenerative Diseases Linked to Mitochondrial Impairment
Defects in mitochondrial function have now been linked to many of the most common diseases of the aged population. These include Type II Diabetes Mellitus, Parkinson Disease, Atherosclerotic Heart Disease, Stroke, Alzheimer Dementia, and Cancer. While it cannot yet be said that mitochondria are the only cause of these problems, it is clear that mitochondria are involved because their function is “measurably” disturbed. Even autoimmune diseases such as Multiple Sclerosis, Systemic Lupus Erythematosus, and Rheumatoid Arthritis appear to have mitochondrial components. Each time a disease is investigated, mitochondrial impairment links are found.
Mitochondria normally become severely impaired with age. This impairment results in high levels of free radicals that not only continually damage the mitochondria, but other important parts of the cell (DNA), leading to a vicious downward spiral in overall cell function. Mitochondrial decay may also result in energy deficits and an inability to dispose of toxins from the environment and may cause cells to die prematurely. Results led to the conclusion that mitochondria can be called the "Achilles Heel" of the cell in terms of aging.
Researchers are now wondering if mitochondrial impairment might be at the heart of many more diseases and disorders.
Primary defects in mitochondrial function are implicated in over 100 diseases, and the list continues to grow. Yet, the first mitochondrial defect--a myopathy--was demonstrated only 35 years ago.
With increased understanding of the mechanisms, underlying mitochondrial dysfunctions came the beginnings of therapeutic strategies, based mostly on the administration of antioxidants, replacement of cofactors, vitamins and minerals, hormone balancing, and provision of nutrients. At the present accelerating pace of development of what may be called mitochondrial medicine, much more is likely to be achieved within the next few years.
In 1995, the entire program of the 25th annual meeting of the American Aging Association and the American College of Clinical Gerontology was devoted to the role of mitochondria in the chronic diseases. Even so, most physicians in America are not yet aware of the connection between chronic diseases and abnormalities in mitochondrial function. Fortunately, this is changing through education. However, real change in medicine requires two elements - education and the availability of effective treatment. Progress will be slow until effective treatments for mitochondrial diseases are developed.
The Really Good News? The mitochondria processes related to aging and premature aging are highly adaptable with different (individual) interventions. What’s more, these processes can be measured with special laboratory tests. Moreover, you CAN make use of this knowledge to make significant changes that will and do help you to maintain your current good health or assist in reversing current health and pain causing conditions – including back and neck pain conditions.
Are We Aging Prematurely?
According to the federal government, Americans live an average of 73.7 years, but spend their last 11.7 years in "dysfunctional life", which is marked by disease and impairment. This must mean that aging factors are beginning to create degenerative disease changes in our bodies long before the age of 62. In fact, studies have shown many degenerative disease processes begin in a person’s 20s. [1] Remember, this age of 73.7 years is an “average.” This must then mean that there are a good number of people developing degenerative diseases at even younger ages then 62. Centenarian societies are still physically active, healthy, and happy long into their 80s – 90s while showing little if any western degenerative diseases, even into these ages. Consider that studies have determined (without problematic genetic aspects) humans are biologically “set” to live well into their 120th year.
When looking at aging and premature aging research we compiled a list of four scientifically well accepted contributors to the aging process. They are:
IMPAIRED MITOCHONDRIAL FUNCTION
1a) Oxidative Stress
1b) Impaired or abnormal fluctuations in levels of
blood sugar and insulin (Dysglycemia)
1c) Increased combining of glucose and
proteins (Glycation)
1) IMPAIRED MITOCHONDRIAL FUNCTION
A quick look at the above list and it is easy to see we did not include 1a to 1c as primary factors. That is because while they are contributors to aging and pre-mature aging in their own right, they are also the known prime contributors to impaired mitochondrial and cellular function. Impaired mitochondrial functioning is looking more and more like one of the crucial candidates for all the symptoms of premature aging and, by extension, over 100% of the leading causes of death by disease and the various conditions, syndromes and symptoms that accompany these diseases plaguing western health.
While we are certain many more aspects of the body’s interacting metabolic systems will be implicated as causative of premature aging and the aged, this article looks at the known factors about mitochondrial functioning that we have control over NOW in our quest for homeostasis, health and relief from needless pain.
Why are Mitochondria so Important?
The mitochondria (the plural of Mitochondrion) are commonly referred to as the "powerhouses" of the cell. Every cell throughout our body contains a number of these little “powerhouses”. Depending upon the specific type of cell there can be one or thousands of mitochondrion in each cell.
Mitochondria consume over 80 percent of the oxygen we breathe and make over 90 percent of the energy our cells need to function. They use the oxygen in the air we breathe to release energy from food. This process transforms food calories into chemical energy, water, and carbon dioxide. The released chemical energy is then stored in the form of adenosine triphosphate (ATP). ATP is the universal currency of energy used by all life on earth. It is like an electrical power source that drives the engines of the cell. This process of burning food to make ATP is called oxidative phosphorylation. Only mitochondria can do it. Without it, muscles could not contract and neurons could not fire. Mitochondria literally make it possible for us to move and think.
Production of ATP is far from the only major function these infinitely tiny cellular components use to help keep vital homeostasis on the go.
Some mitochondrial functions are performed only in specific types of cells. For example, mitochondria in liver cells contain enzymes that allow them to detoxify ammonia, a waste product of protein metabolism. These enzymes are not made in the mitochondria of cardiac cells. As more research is done greater information about their role is being revealed. What is known is just the “tip of the iceberg” thing again.
What Are Some of Their Other Functions?
Here is what we know today about some of the other functions these stupendously tiny cellular components have a role in:
apoptosis (normal cell death)
Glutamate-mediated excitotoxic neuronal (Nerve) injury
Cellular reproduction
heme (iron) synthesis
steroid synthesis * - Also called steroid hormones. Synthesis of all steroids must have cholesterol to begin. (*Any of numerous naturally occurring fat-soluble organic compounds such as plant sterols and bile acids, adrenocortical and sex hormones, and the precursors of certain vitamins.)
heat production (enabling the organism to stay warm)
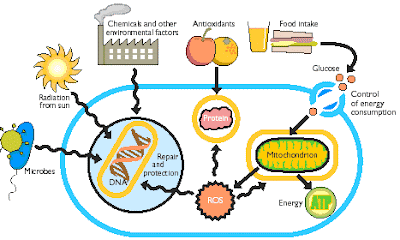
Mitochondria are important in relation to the aging process for two reasons: their role in energy production and, the generation of free radicals. Yes, these little powerhouses are radically responsible for the production of free radicals in the body.
1a) Oxidative Stress (Caused by Free Radicals)
Although we cannot survive without oxygen, oxygen also contributes to our death by forming free radicals, which are unstable and highly reactive molecules that alter metabolism and damage physical structures. The mitochondria are the major sites within the body for the production of free radicals. In a healthy state, about 20% of the oxygen we breathe forms free radicals. In an unhealthy state up to 80% of the oxygen we breath forms free radicals.
When the mitochondria are malfunctioning, more free radicals are formed. Chemical, Metal, Drug Toxicity, nutrient inadequacy, and stress adversely affect mitochondrial function by adding to increased free radical production.
Mitochondria possess their own DNA (genetic material), which unlike the DNA housed in the nucleus (center of the cell) is very vulnerable to mutation (damage) from oxidative stress (free radicals). When mutation of this DNA occurs, mitochondrial function may become severely damaged. It is easy to get into a cycle of poor mitochondrial function, oxidative stress, mitochondrial mutation, further impairment of mitochondrial function and so on.
Not only does mitochondrial impairment contribute to aging by increasing free radical production, but reduced generation of energy plays a role as well. We all undergo degenerative changes as a result of normal aging; loss of normal structure and function, as our cells "run out of gas" due to mitochondrial failure. Degeneration and eventually death results.
While our nuclear (found in the nucleus (center) of the cell) DNA is wonderfully protected, unfortunately we seem stuck with a poor mitochondrial DNA repair capacity. Each mitochondrion’s DNA can take only so many free-radical "hits." For instance, mitochondrial malfunction within brain cells results in cognitive impairment and death of those cells, contributing to the likes of Alzheimer and Parkinson’s diseases.
Mitochondrial impairment is often first observed in the cells that contain greater numbers of mitochondria: like the cells of the heart, brain, muscles, liver, immune system, and gastrointestinal lining.
What causes this “Oxidative Stress”?
Common sources of oxidative stress are: toxicity, chronic inflammation, glycation, stress, excessive exercise, some medications, alcohol, cigarette smoke, and dietary factors such as consumption of refined carbohydrates (white sugar and flour, for a start), bad fat, bad oils, fried oils, and foods cooked at high temperature (fried or barbequed for example). Overeating may be another source of oxidative stress. The more food that is burned within the mitochondria the more free radicals are produced. Animal studies have actually demonstrated that caloric restriction extends lifespan.
These are the same scientifically identified Major Contributors to aging, premature aging and degenerative disease development, which we discussed in our article on Degenerative Conditions of the Back and Neck.
What protects and assists mitochondrial function?
Many nutrients are critical for proper mitochondrial function and protection from oxidative stress. The diet needs to supply quality proteins, fats and oils, carbohydrates, and an abundance of antioxidants. And, this diet must be ingested in a healthy ratio of protein to fats to carbs. Certainly none of this is to be found in the Standard American Diet (Acronym: SAD)
Antioxidants are found in abundance in unprocessed, fresh, fully matured plant foods, grown in healthy soil.
Supplemental nutrients of particular importance to mitochondrial function include coenzyme Q10, R-alpha lipoic acid, L-Acetyl Carnitine (AKA: Acetyl-L-Carnitine), and vitamin E.
Aerobic, weight bearing, and limbic exercise (no excessive exercise) also improves mitochondrial function and increases the body’s antioxidant defenses.
There are many other nutrients and supplements that can be of assistance and may be needed over and above the healthy diet and those listed above. We have left them out of this list on purpose. They should only be used after proper testing has been done, and prescribed only under the care of a natural physician or a medical model specialist in endocrinology and mitochondrial disease. For example: people diagnoses as diabetic II, can find their blood sugar reduced to dangerously low levels with the use of R-ALA and L-Acetyl Carnitine when they are also taking pharmaceutical medications that seek to produce the same result.
Testing for Mitochondrial Health
Two particularly good tests for assessing mitochondrial health are the Oxidative Stress Analysis and the Organic Acid Analysis. Information from both tests can be used to tailor therapy for the individual to improve mitochondrial function. These are tests available through order of a Natural doctor. Some times under special circumstances an MD may be able to order such tests, but it is not a usual practice.
The Oxidative Stress Analysis is a laboratory test performed on blood and urine specimens to evaluate levels of oxidative stress and adequacy of the body’s antioxidants.
The Organic Acid Analysis utilizes a urine sample to measure concentrations of compounds produced by the body’s metabolism. Several of these organic acids are produced within mitochondria. Abnormal levels of mitochondria-derived organic acids signify altered mitochondrial function.
1b) Blood Sugar Fluctuations (Dysclycemia)
Dysglycemia is basically abnormal fluctuations in levels of blood sugar resulting in high levels of insulin. Another term is Insulin Resistance. Dysglycemia accelerates the aging process in a number of ways. It impairs mitochondrial functioning and raises levels of insulin, which contributes to obesity, hypertension, atherosclerosis, and accelerated tumor growth and produces pro-aging imbalances in other hormones. Some of these hormonal abnormalities in turn lead to further dysglycemia. Dysglycemia impairs immune function, increases inflammation and leads to increased protein glycation. Dysglycemia is the metabolic precursor of adult-onset diabetes. Diabetes complications include vascular and heart disease, eye, nerve and kidney damage and other conditions.
1c) Protein Glycation
Glycation is the combining of glucose with proteins, and it occurs continuously throughout the body. The higher the levels of glucose in the blood and the longer they stay elevated, the more glycation occurs. Insulin resistance, which results from prolonged dysglycemia, allows blood glucose to rise to abnormal levels. Glycation results in altered structure and function of the protein. Glycation also contributes to inflammation and to increased oxidative stress and mitochondrial damage. The rate of protein glycation has been found to correlate with biological aging.
COOKING AT HIGH TEMPERATURES
Eating foods cooked at high temperatures creates glycation and this is another contributor to the production of inflammatory cytokines. In fact, it has been shown that eating such foods leads to the formation of advanced glycation end products. (AGE’s) Glycation can be described as the binding of a protein molecule to a glucose molecule, resulting in the formation of damaged protein structures. Many age-related diseases such as arterial stiffening, cataracts, and neurological impairment are at least partially attributable to glycation. These destructive glycation reactions render proteins in the body cross-linked and barely functional. As these degraded proteins accumulate, they cause cells to emit signals that induce the production of inflammatory cytokines (such as IL-6 and TNF-a)
History and Current Knowledge of Mitochondria
Mitochondrial medicine is a new and rapidly developing medical subspecialty. Many specialists are now involved in researching mitochondrial diseases, including physicians specializing in metabolic diseases, cell biologists, molecular geneticists, neurologists, biochemists, pathologists, immunologists, and embryologists. Natural Model scientist are also looking at and participating in this research. For them it has provided yet more scientific proof that the body works as a single interrelated unit.
Studies linking mitochondrial damage to the aging processes are the result of discovery of genetic mitochondria abnormalities “disorders” in children. The first patient was diagnosed with a genetic mitochondrial disorder in 1959. One thousand to 4,000 children per year are born with a type of mitochondrial “disease” in the United Sates.
Many of the “diseases” associated with “aging” that would normally begin to appear in people after the age of 50 to 70 showed mitochondrial damage similar to (disease) disorders suffered by those children. Among these, but not limited to, are type 2 diabetes, Parkinson's disease, atherosclerotic heart disease, stroke, Alzheimer's disease and cancer. Once aware of this damage, which was normal in the aged, they began to find this same mitochondrial damage and it’s expected diseases occuring in a much younger population, as well. This is now referred to as pre-mature aging.
The additional discovery that many medications can injure the mitochondria then led research into evaluating how other toxic environmental factors cause dysfunctional mitochondria and lead to “diseases.” These include many of the 80,000 chemicals produced by industry including artificial hormones and metals.
Mitochondrial dysfunction can affect every part of the body. In some people only one organ may be involved, in others all organs may be affected. However, we want to make the strong point that no matter what – a problem in one area (organ or system) sooner or later upsets everything else. The length of time this takes before outward, undeniable physical symptoms occur depends on too many factors to evaluate here. However, this internal upset DOES occur and WILL produce ever more negative results to health. The longer one takes in starting the processes that will identify and begin to rectify such dysfunctions the greater the danger to develop more complicated disease conditions.
Symptoms Associated with Impaired Mitochondria
Medical model science supplies a list of symptoms associated with impaired and damaged mitochondria. After reviewing these we hope you agree that they make it easy to see that this list links to, and encompasses, all major health problems in western world lifestyle countries.
Their list is as follows:
{Quote} Depending on which specific cells of the body are affected, i.e. liver, heart, brain, etc., mitochondrial impairment or dysfunction within these cells may include symptoms such as:
• unrelieved fatigue
• Poor growth
• Loss of muscle coordination, muscle weakness
• Visual and/or hearing problems
• Developmental delays, learning disabilities
• Mental retardation
• Heart, liver or kidney disease
• Gastrointestinal disorders, severe constipation
• Respiratory disorders
• Diabetes
• Immune system dysfunction (Increased risk of infection, inflammation, cancer)
• Neurological problems, seizures
• Thyroid dysfunction
• Steroid dysfunction
• Dementia (mental disorder characterized by confusion, disorientation and memory loss) {Unquote}
Insulin and aging
Centenarians, people who have lived over 100 years, don't have much in common. For example, some are smokers – others not. They come from all over the world without a favoring any geographic location in particular.
However, there are 3 consistent metabolic blood indicators common to all centenarians; low blood sugar, low insulin, and low triglycerides. All 3 are relatively low vs. chronological age. Among these 3 indicators/factors insulin is the common denominator.
The level of insulin sensitivity of the cell is one of
the most important markers of lifespan.
Controlling your blood insulin level is one of the most powerful anti-aging strategies you can possibly put into action.
Insulin Resistance is being extensively studied by endocrinologists. So far, these studies have linked insulin resistance with almost every life-threatening disease, and their complications - and they are still looking. In many cases the link is direct (causative) to the disease, in other cases it is a result or cause of a complication of the disease. Metabolic Syndrome (definitely linked to Insulin Resistance) was first identified as a group of symptoms indicating a high risk for heart and vascular disease and diabetes II – but it was only a part of an overall investigation in the possible links between insulin resistance and any or all diseases. Both Metabolic Syndrome, and the Metabolic Affects of Westernized Living, looks at Insulin Resistance’s connection to Aging and Premature Aging diseases. When we consider the information we have just read – it certainly looks like the sugar/insulin problem is ONE of the big reasons we have increasing poor health – a slippery slope to the development of all the diseases associated with impaired mitochondrial function and premature aging for sure. And it may turn out to be the biggest cause. It is also the easiest one to change.
In Conclusion:
No matter which model of medicine you favor for your back or neck pain care and treatment we ask you to keep in mind there is no doubt the body does not work as independent parts. What affects one part affects every part. Health, and a healthy, pain free body, begin and end at the cellular-chemical level. It would seem that the most important part of the cellular-chemical health or metabolic disease process that we have the power to change, lies within our mitochondria. Thorough individualized tests are required to determine an overall metabolic imbalance and thereby where to begin rebalancing. We ask you to remember that orthodox testing cannot give you such definitive answers.
References:
[1] Cheryl Duxbury. Aging and the Threshold of Disease, University of Waterloo, CA. Course Notes Biology and Human Aging
R Luft, Rolf Luft Research Institute, Department of Molecular Medicine, Karolinska Hospital, Stockholm, Sweden “The development of mitochondrial medicine.” Proc Natl Acad Sci U S A. 1994 September 13; 91(19): 8731–8738.
Joseph Debe, MD, "A Scientific Approach to Anti-Aging", www.drdebe.com/antiage.htm
Trifunovic, A. et al. (2004). “Premature ageing in mice expressing defective mitochondrial DNA polymerase.” Nature; 429(6990): 417–23.
Cadenas E, Davies KJ. "Mitochondrial free radical generation, oxidative stress, and aging." Free Radic Biol Med 2000 Aug;29(3-4):222-30
Ebadi M. "Introduction. Oxidative stress in mitochondria disorders of aging." Biol Signals Recept 2001 Jan-Apr;10(1-2):5-13
Kowald A. "The mitochondrial theory of aging." Biol Signals Recept 2001 May-Aug;10(3-4):162-75
Allen JF, Allen CA. "A mitochondrial model for premature aging of somatically cloned mammals." IUBMB Life 1999 Oct;48(4):369-72
Van Remmen H, Richardson A. "Oxidative damage to mitochondria and aging." Exp Gerontol 2001 Jul;36(7):957-68
Wei YH, Lu CY, Wei CY, Ma YS, Lee HC. "Oxidative stress in human aging and mitochondrial disease-consequences of defective mitochondrial respiration and impaired antioxidant enzyme system," Chin J Physiol 2001 Mar 31;44(1):1-11
“Accumulation of deletions and point mutations in mitochondrial genome in degenerative diseases.” M. Tanaka, S. A. Kovalenko, J. S. Gong, H. J. Borgeld, K. Katsumata, M. Hayakawa, M. Yoneda and T. Ozawa Department of Biomedical Chemistry, Faculty of Medicine, University of Nagoya, Japan
“Mitochondrial Medicine – Molecular Pathology of Defective Oxidative Phosphorylation. ” Egil Fosslien
Aubrey de Grey. “The Mitochondrial Free Radical Theory of Aging.” (R.G. Landes Co, 1999).
Aubrey de Grey. "The reductive hotspot hypothesis of mammalian aging: Membrane metabolism magnifies mutant mitochondrial mischief," European Journal of Biochemistry. Vol 269 : pp.2003-2009 (Apr 2002) (Minireview).
Douglas C. Wallace. "Mitochondrial Diseases in Man and Mouse," Science. pp. 1482-1488 (5 March 1999)
Douglas C. Wallace. "Mitochondrial DNA in Aging and Disease," Scientific American. pp. 40-47 (August 1997)
U.T. Brunk and A. Terman. "The mitochondrial-lysosomal axis theory of aging: Accumulation of damaged mitochondria as a result of imperfect autophagocytosis," European Journal of Biochemistry. Vol 269 : pp.1996-2002 (Apr 2002) (Minireview).
Makiko S. Fliss, et.al. "Facile Detection of Mitochondrial DNA Mutations in Tumors and Bodily Fluids," Science. pp. 2017-2019 (17 March 2000)
There are many theories of aging. Some involve processes over which we presently have no control and a huge number yet undiscovered. Out of this highly contentious issue arose a theory involving mitochondria in our cells, which shows the greatest promise to modify, reverse, and “cure” degenerative diseases. Mitochondrial Medicine may prove to be the wave of the future for medicine. It is already proving of benefit in returning homeostasis to “disorders” in the body. Mitochondrial impairment has been linked to the degenerative diseases of normal aging and because these same impairments are also being found in younger aged people they are thought to be the cause of “premature aging” and therefore why younger people are developing these diseases of the aged. Identified factors that lead to mitochondrial impairment just happen to be factors that are adaptable to change by ourselves and with the help of sophisticated metabolic testing.
The Degenerative Diseases Linked to Mitochondrial Impairment
Defects in mitochondrial function have now been linked to many of the most common diseases of the aged population. These include Type II Diabetes Mellitus, Parkinson Disease, Atherosclerotic Heart Disease, Stroke, Alzheimer Dementia, and Cancer. While it cannot yet be said that mitochondria are the only cause of these problems, it is clear that mitochondria are involved because their function is “measurably” disturbed. Even autoimmune diseases such as Multiple Sclerosis, Systemic Lupus Erythematosus, and Rheumatoid Arthritis appear to have mitochondrial components. Each time a disease is investigated, mitochondrial impairment links are found.
Mitochondria normally become severely impaired with age. This impairment results in high levels of free radicals that not only continually damage the mitochondria, but other important parts of the cell (DNA), leading to a vicious downward spiral in overall cell function. Mitochondrial decay may also result in energy deficits and an inability to dispose of toxins from the environment and may cause cells to die prematurely. Results led to the conclusion that mitochondria can be called the "Achilles Heel" of the cell in terms of aging.
Researchers are now wondering if mitochondrial impairment might be at the heart of many more diseases and disorders.
Primary defects in mitochondrial function are implicated in over 100 diseases, and the list continues to grow. Yet, the first mitochondrial defect--a myopathy--was demonstrated only 35 years ago.
With increased understanding of the mechanisms, underlying mitochondrial dysfunctions came the beginnings of therapeutic strategies, based mostly on the administration of antioxidants, replacement of cofactors, vitamins and minerals, hormone balancing, and provision of nutrients. At the present accelerating pace of development of what may be called mitochondrial medicine, much more is likely to be achieved within the next few years.
In 1995, the entire program of the 25th annual meeting of the American Aging Association and the American College of Clinical Gerontology was devoted to the role of mitochondria in the chronic diseases. Even so, most physicians in America are not yet aware of the connection between chronic diseases and abnormalities in mitochondrial function. Fortunately, this is changing through education. However, real change in medicine requires two elements - education and the availability of effective treatment. Progress will be slow until effective treatments for mitochondrial diseases are developed.
The Really Good News? The mitochondria processes related to aging and premature aging are highly adaptable with different (individual) interventions. What’s more, these processes can be measured with special laboratory tests. Moreover, you CAN make use of this knowledge to make significant changes that will and do help you to maintain your current good health or assist in reversing current health and pain causing conditions – including back and neck pain conditions.
Are We Aging Prematurely?
According to the federal government, Americans live an average of 73.7 years, but spend their last 11.7 years in "dysfunctional life", which is marked by disease and impairment. This must mean that aging factors are beginning to create degenerative disease changes in our bodies long before the age of 62. In fact, studies have shown many degenerative disease processes begin in a person’s 20s. [1] Remember, this age of 73.7 years is an “average.” This must then mean that there are a good number of people developing degenerative diseases at even younger ages then 62. Centenarian societies are still physically active, healthy, and happy long into their 80s – 90s while showing little if any western degenerative diseases, even into these ages. Consider that studies have determined (without problematic genetic aspects) humans are biologically “set” to live well into their 120th year.
When looking at aging and premature aging research we compiled a list of four scientifically well accepted contributors to the aging process. They are:
IMPAIRED MITOCHONDRIAL FUNCTION
1a) Oxidative Stress
1b) Impaired or abnormal fluctuations in levels of
blood sugar and insulin (Dysglycemia)
1c) Increased combining of glucose and
proteins (Glycation)
1) IMPAIRED MITOCHONDRIAL FUNCTION
A quick look at the above list and it is easy to see we did not include 1a to 1c as primary factors. That is because while they are contributors to aging and pre-mature aging in their own right, they are also the known prime contributors to impaired mitochondrial and cellular function. Impaired mitochondrial functioning is looking more and more like one of the crucial candidates for all the symptoms of premature aging and, by extension, over 100% of the leading causes of death by disease and the various conditions, syndromes and symptoms that accompany these diseases plaguing western health.
While we are certain many more aspects of the body’s interacting metabolic systems will be implicated as causative of premature aging and the aged, this article looks at the known factors about mitochondrial functioning that we have control over NOW in our quest for homeostasis, health and relief from needless pain.
Why are Mitochondria so Important?
The mitochondria (the plural of Mitochondrion) are commonly referred to as the "powerhouses" of the cell. Every cell throughout our body contains a number of these little “powerhouses”. Depending upon the specific type of cell there can be one or thousands of mitochondrion in each cell.
Mitochondria consume over 80 percent of the oxygen we breathe and make over 90 percent of the energy our cells need to function. They use the oxygen in the air we breathe to release energy from food. This process transforms food calories into chemical energy, water, and carbon dioxide. The released chemical energy is then stored in the form of adenosine triphosphate (ATP). ATP is the universal currency of energy used by all life on earth. It is like an electrical power source that drives the engines of the cell. This process of burning food to make ATP is called oxidative phosphorylation. Only mitochondria can do it. Without it, muscles could not contract and neurons could not fire. Mitochondria literally make it possible for us to move and think.
Production of ATP is far from the only major function these infinitely tiny cellular components use to help keep vital homeostasis on the go.
Some mitochondrial functions are performed only in specific types of cells. For example, mitochondria in liver cells contain enzymes that allow them to detoxify ammonia, a waste product of protein metabolism. These enzymes are not made in the mitochondria of cardiac cells. As more research is done greater information about their role is being revealed. What is known is just the “tip of the iceberg” thing again.
What Are Some of Their Other Functions?
Here is what we know today about some of the other functions these stupendously tiny cellular components have a role in:
apoptosis (normal cell death)
Glutamate-mediated excitotoxic neuronal (Nerve) injury
Cellular reproduction
heme (iron) synthesis
steroid synthesis * - Also called steroid hormones. Synthesis of all steroids must have cholesterol to begin. (*Any of numerous naturally occurring fat-soluble organic compounds such as plant sterols and bile acids, adrenocortical and sex hormones, and the precursors of certain vitamins.)
heat production (enabling the organism to stay warm)
Mitochondria are important in relation to the aging process for two reasons: their role in energy production and, the generation of free radicals. Yes, these little powerhouses are radically responsible for the production of free radicals in the body.
1a) Oxidative Stress (Caused by Free Radicals)
Although we cannot survive without oxygen, oxygen also contributes to our death by forming free radicals, which are unstable and highly reactive molecules that alter metabolism and damage physical structures. The mitochondria are the major sites within the body for the production of free radicals. In a healthy state, about 20% of the oxygen we breathe forms free radicals. In an unhealthy state up to 80% of the oxygen we breath forms free radicals.
When the mitochondria are malfunctioning, more free radicals are formed. Chemical, Metal, Drug Toxicity, nutrient inadequacy, and stress adversely affect mitochondrial function by adding to increased free radical production.
Mitochondria possess their own DNA (genetic material), which unlike the DNA housed in the nucleus (center of the cell) is very vulnerable to mutation (damage) from oxidative stress (free radicals). When mutation of this DNA occurs, mitochondrial function may become severely damaged. It is easy to get into a cycle of poor mitochondrial function, oxidative stress, mitochondrial mutation, further impairment of mitochondrial function and so on.
Not only does mitochondrial impairment contribute to aging by increasing free radical production, but reduced generation of energy plays a role as well. We all undergo degenerative changes as a result of normal aging; loss of normal structure and function, as our cells "run out of gas" due to mitochondrial failure. Degeneration and eventually death results.
While our nuclear (found in the nucleus (center) of the cell) DNA is wonderfully protected, unfortunately we seem stuck with a poor mitochondrial DNA repair capacity. Each mitochondrion’s DNA can take only so many free-radical "hits." For instance, mitochondrial malfunction within brain cells results in cognitive impairment and death of those cells, contributing to the likes of Alzheimer and Parkinson’s diseases.
Mitochondrial impairment is often first observed in the cells that contain greater numbers of mitochondria: like the cells of the heart, brain, muscles, liver, immune system, and gastrointestinal lining.
What causes this “Oxidative Stress”?
Common sources of oxidative stress are: toxicity, chronic inflammation, glycation, stress, excessive exercise, some medications, alcohol, cigarette smoke, and dietary factors such as consumption of refined carbohydrates (white sugar and flour, for a start), bad fat, bad oils, fried oils, and foods cooked at high temperature (fried or barbequed for example). Overeating may be another source of oxidative stress. The more food that is burned within the mitochondria the more free radicals are produced. Animal studies have actually demonstrated that caloric restriction extends lifespan.
These are the same scientifically identified Major Contributors to aging, premature aging and degenerative disease development, which we discussed in our article on Degenerative Conditions of the Back and Neck.
What protects and assists mitochondrial function?
Many nutrients are critical for proper mitochondrial function and protection from oxidative stress. The diet needs to supply quality proteins, fats and oils, carbohydrates, and an abundance of antioxidants. And, this diet must be ingested in a healthy ratio of protein to fats to carbs. Certainly none of this is to be found in the Standard American Diet (Acronym: SAD)
Antioxidants are found in abundance in unprocessed, fresh, fully matured plant foods, grown in healthy soil.
Supplemental nutrients of particular importance to mitochondrial function include coenzyme Q10, R-alpha lipoic acid, L-Acetyl Carnitine (AKA: Acetyl-L-Carnitine), and vitamin E.
Aerobic, weight bearing, and limbic exercise (no excessive exercise) also improves mitochondrial function and increases the body’s antioxidant defenses.
There are many other nutrients and supplements that can be of assistance and may be needed over and above the healthy diet and those listed above. We have left them out of this list on purpose. They should only be used after proper testing has been done, and prescribed only under the care of a natural physician or a medical model specialist in endocrinology and mitochondrial disease. For example: people diagnoses as diabetic II, can find their blood sugar reduced to dangerously low levels with the use of R-ALA and L-Acetyl Carnitine when they are also taking pharmaceutical medications that seek to produce the same result.
Testing for Mitochondrial Health
Two particularly good tests for assessing mitochondrial health are the Oxidative Stress Analysis and the Organic Acid Analysis. Information from both tests can be used to tailor therapy for the individual to improve mitochondrial function. These are tests available through order of a Natural doctor. Some times under special circumstances an MD may be able to order such tests, but it is not a usual practice.
The Oxidative Stress Analysis is a laboratory test performed on blood and urine specimens to evaluate levels of oxidative stress and adequacy of the body’s antioxidants.
The Organic Acid Analysis utilizes a urine sample to measure concentrations of compounds produced by the body’s metabolism. Several of these organic acids are produced within mitochondria. Abnormal levels of mitochondria-derived organic acids signify altered mitochondrial function.
1b) Blood Sugar Fluctuations (Dysclycemia)
Dysglycemia is basically abnormal fluctuations in levels of blood sugar resulting in high levels of insulin. Another term is Insulin Resistance. Dysglycemia accelerates the aging process in a number of ways. It impairs mitochondrial functioning and raises levels of insulin, which contributes to obesity, hypertension, atherosclerosis, and accelerated tumor growth and produces pro-aging imbalances in other hormones. Some of these hormonal abnormalities in turn lead to further dysglycemia. Dysglycemia impairs immune function, increases inflammation and leads to increased protein glycation. Dysglycemia is the metabolic precursor of adult-onset diabetes. Diabetes complications include vascular and heart disease, eye, nerve and kidney damage and other conditions.
1c) Protein Glycation
Glycation is the combining of glucose with proteins, and it occurs continuously throughout the body. The higher the levels of glucose in the blood and the longer they stay elevated, the more glycation occurs. Insulin resistance, which results from prolonged dysglycemia, allows blood glucose to rise to abnormal levels. Glycation results in altered structure and function of the protein. Glycation also contributes to inflammation and to increased oxidative stress and mitochondrial damage. The rate of protein glycation has been found to correlate with biological aging.
COOKING AT HIGH TEMPERATURES
Eating foods cooked at high temperatures creates glycation and this is another contributor to the production of inflammatory cytokines. In fact, it has been shown that eating such foods leads to the formation of advanced glycation end products. (AGE’s) Glycation can be described as the binding of a protein molecule to a glucose molecule, resulting in the formation of damaged protein structures. Many age-related diseases such as arterial stiffening, cataracts, and neurological impairment are at least partially attributable to glycation. These destructive glycation reactions render proteins in the body cross-linked and barely functional. As these degraded proteins accumulate, they cause cells to emit signals that induce the production of inflammatory cytokines (such as IL-6 and TNF-a)
History and Current Knowledge of Mitochondria
Mitochondrial medicine is a new and rapidly developing medical subspecialty. Many specialists are now involved in researching mitochondrial diseases, including physicians specializing in metabolic diseases, cell biologists, molecular geneticists, neurologists, biochemists, pathologists, immunologists, and embryologists. Natural Model scientist are also looking at and participating in this research. For them it has provided yet more scientific proof that the body works as a single interrelated unit.
Studies linking mitochondrial damage to the aging processes are the result of discovery of genetic mitochondria abnormalities “disorders” in children. The first patient was diagnosed with a genetic mitochondrial disorder in 1959. One thousand to 4,000 children per year are born with a type of mitochondrial “disease” in the United Sates.
Many of the “diseases” associated with “aging” that would normally begin to appear in people after the age of 50 to 70 showed mitochondrial damage similar to (disease) disorders suffered by those children. Among these, but not limited to, are type 2 diabetes, Parkinson's disease, atherosclerotic heart disease, stroke, Alzheimer's disease and cancer. Once aware of this damage, which was normal in the aged, they began to find this same mitochondrial damage and it’s expected diseases occuring in a much younger population, as well. This is now referred to as pre-mature aging.
The additional discovery that many medications can injure the mitochondria then led research into evaluating how other toxic environmental factors cause dysfunctional mitochondria and lead to “diseases.” These include many of the 80,000 chemicals produced by industry including artificial hormones and metals.
Mitochondrial dysfunction can affect every part of the body. In some people only one organ may be involved, in others all organs may be affected. However, we want to make the strong point that no matter what – a problem in one area (organ or system) sooner or later upsets everything else. The length of time this takes before outward, undeniable physical symptoms occur depends on too many factors to evaluate here. However, this internal upset DOES occur and WILL produce ever more negative results to health. The longer one takes in starting the processes that will identify and begin to rectify such dysfunctions the greater the danger to develop more complicated disease conditions.
Symptoms Associated with Impaired Mitochondria
Medical model science supplies a list of symptoms associated with impaired and damaged mitochondria. After reviewing these we hope you agree that they make it easy to see that this list links to, and encompasses, all major health problems in western world lifestyle countries.
Their list is as follows:
{Quote} Depending on which specific cells of the body are affected, i.e. liver, heart, brain, etc., mitochondrial impairment or dysfunction within these cells may include symptoms such as:
• unrelieved fatigue
• Poor growth
• Loss of muscle coordination, muscle weakness
• Visual and/or hearing problems
• Developmental delays, learning disabilities
• Mental retardation
• Heart, liver or kidney disease
• Gastrointestinal disorders, severe constipation
• Respiratory disorders
• Diabetes
• Immune system dysfunction (Increased risk of infection, inflammation, cancer)
• Neurological problems, seizures
• Thyroid dysfunction
• Steroid dysfunction
• Dementia (mental disorder characterized by confusion, disorientation and memory loss) {Unquote}
Insulin and aging
Centenarians, people who have lived over 100 years, don't have much in common. For example, some are smokers – others not. They come from all over the world without a favoring any geographic location in particular.
However, there are 3 consistent metabolic blood indicators common to all centenarians; low blood sugar, low insulin, and low triglycerides. All 3 are relatively low vs. chronological age. Among these 3 indicators/factors insulin is the common denominator.
The level of insulin sensitivity of the cell is one of
the most important markers of lifespan.
Controlling your blood insulin level is one of the most powerful anti-aging strategies you can possibly put into action.
Insulin Resistance is being extensively studied by endocrinologists. So far, these studies have linked insulin resistance with almost every life-threatening disease, and their complications - and they are still looking. In many cases the link is direct (causative) to the disease, in other cases it is a result or cause of a complication of the disease. Metabolic Syndrome (definitely linked to Insulin Resistance) was first identified as a group of symptoms indicating a high risk for heart and vascular disease and diabetes II – but it was only a part of an overall investigation in the possible links between insulin resistance and any or all diseases. Both Metabolic Syndrome, and the Metabolic Affects of Westernized Living, looks at Insulin Resistance’s connection to Aging and Premature Aging diseases. When we consider the information we have just read – it certainly looks like the sugar/insulin problem is ONE of the big reasons we have increasing poor health – a slippery slope to the development of all the diseases associated with impaired mitochondrial function and premature aging for sure. And it may turn out to be the biggest cause. It is also the easiest one to change.
In Conclusion:
No matter which model of medicine you favor for your back or neck pain care and treatment we ask you to keep in mind there is no doubt the body does not work as independent parts. What affects one part affects every part. Health, and a healthy, pain free body, begin and end at the cellular-chemical level. It would seem that the most important part of the cellular-chemical health or metabolic disease process that we have the power to change, lies within our mitochondria. Thorough individualized tests are required to determine an overall metabolic imbalance and thereby where to begin rebalancing. We ask you to remember that orthodox testing cannot give you such definitive answers.
References:
[1] Cheryl Duxbury. Aging and the Threshold of Disease, University of Waterloo, CA. Course Notes Biology and Human Aging
R Luft, Rolf Luft Research Institute, Department of Molecular Medicine, Karolinska Hospital, Stockholm, Sweden “The development of mitochondrial medicine.” Proc Natl Acad Sci U S A. 1994 September 13; 91(19): 8731–8738.
Joseph Debe, MD, "A Scientific Approach to Anti-Aging", www.drdebe.com/antiage.htm
Trifunovic, A. et al. (2004). “Premature ageing in mice expressing defective mitochondrial DNA polymerase.” Nature; 429(6990): 417–23.
Cadenas E, Davies KJ. "Mitochondrial free radical generation, oxidative stress, and aging." Free Radic Biol Med 2000 Aug;29(3-4):222-30
Ebadi M. "Introduction. Oxidative stress in mitochondria disorders of aging." Biol Signals Recept 2001 Jan-Apr;10(1-2):5-13
Kowald A. "The mitochondrial theory of aging." Biol Signals Recept 2001 May-Aug;10(3-4):162-75
Allen JF, Allen CA. "A mitochondrial model for premature aging of somatically cloned mammals." IUBMB Life 1999 Oct;48(4):369-72
Van Remmen H, Richardson A. "Oxidative damage to mitochondria and aging." Exp Gerontol 2001 Jul;36(7):957-68
Wei YH, Lu CY, Wei CY, Ma YS, Lee HC. "Oxidative stress in human aging and mitochondrial disease-consequences of defective mitochondrial respiration and impaired antioxidant enzyme system," Chin J Physiol 2001 Mar 31;44(1):1-11
“Accumulation of deletions and point mutations in mitochondrial genome in degenerative diseases.” M. Tanaka, S. A. Kovalenko, J. S. Gong, H. J. Borgeld, K. Katsumata, M. Hayakawa, M. Yoneda and T. Ozawa Department of Biomedical Chemistry, Faculty of Medicine, University of Nagoya, Japan
“Mitochondrial Medicine – Molecular Pathology of Defective Oxidative Phosphorylation. ” Egil Fosslien
Aubrey de Grey. “The Mitochondrial Free Radical Theory of Aging.” (R.G. Landes Co, 1999).
Aubrey de Grey. "The reductive hotspot hypothesis of mammalian aging: Membrane metabolism magnifies mutant mitochondrial mischief," European Journal of Biochemistry. Vol 269 : pp.2003-2009 (Apr 2002) (Minireview).
Douglas C. Wallace. "Mitochondrial Diseases in Man and Mouse," Science. pp. 1482-1488 (5 March 1999)
Douglas C. Wallace. "Mitochondrial DNA in Aging and Disease," Scientific American. pp. 40-47 (August 1997)
U.T. Brunk and A. Terman. "The mitochondrial-lysosomal axis theory of aging: Accumulation of damaged mitochondria as a result of imperfect autophagocytosis," European Journal of Biochemistry. Vol 269 : pp.1996-2002 (Apr 2002) (Minireview).
Makiko S. Fliss, et.al. "Facile Detection of Mitochondrial DNA Mutations in Tumors and Bodily Fluids," Science. pp. 2017-2019 (17 March 2000)


Comments
Post a Comment